When establishing a dissolution procedure, just one standard aim is to acquire "sink" situations. Sink disorders are defined as the amount of medium that's a minimum of 3 times that necessary to form a saturated Remedy of drug compound.
A well known on the web System direct by pharmaceutical professionals to expand-up pharmaceutical specialists with scientific and complex understanding.
Nonetheless, the look, growth, and validation on the course of action can be pretty included, particularly when 1 considers that not simply need to the dissolution course of action be made and validated, but also any analytical strategy useful for the assay.
Dissolution will be the physicochemical course of action by which a sound compound enters the solvent period to produce an answer.
Several of those injectables or implants rely upon focused drug delivery that incorporate really tiny portions of API. To handle these wants, normal apparatus are already miniaturized to allow dissolution in modest-volume vessels in 50-200 mL or much less, with reciprocating holder apparatus now capable of precise and accurate drug release testing in only five mL.
Filter the sample Resolution immediately via a membrane filter disc with a median pore diameter not better than 1.0 micron. Discard the main handful of ml in the filtrate. Execute the analysis as directed in the person monograph. Repeat The full operation five instances. Where by two or maybe more tablets or capsules are directed being positioned alongside one another from the apparatus, execute 6 replicate tests.
, the disintegration and dissolution of a tablet or capsule is the initial step to therapeutic influence, and control is essential. Dissolution testing provides critical details to assistance the realisation of drug release goals, for evaluating the general performance of various drug substances, for bioequivalence (BE) testing and for product or service QC.
Also, Observe the usage of the time period solubility on The underside axis. With regards to dissolution conduct, we can easily examine both equally the speed of dissolution plus the extent to which the drug is soluble in various media. Both of those are very important.
In-vitro dissolution testing is utilised to get details about the performance of drug solutions since they dissolve. There are numerous types of dissolution apparatus specified by the USP and IP that use distinct mechanisms like baskets, paddles, cylinders or stream-through cells to test dissolution beneath managed temperature and circulation situations.
Some baskets are supplied to be used with no clips, and use a force-on O-ring as a substitute. While they are fantastic For most purposes, it is vital to indicate that the outcome from this layout are the same as the final results obtained with clips - a course of action known as equivalence. It's in no way certain that the outcomes would be the exact same in every single situation.
We make concerted initiatives to make sure that no matter what devices we manufacture and provide they not only arrive up towards the expectations of our valued clients but also stand the test efciency sturdiness & longevity of uninterrupted use.To realize these aims we Be sure that the Uncooked content which we use in our producing approach are of the highest high-quality. Also all instruments are subjected to rigorous top quality control to satisfy correctly the promise furnished by us on our instruments.Eventually with all that we have been dedicated to provide our devices and products and services at quite possibly the most reasonably priced competitive selling prices.We look ahead to carry on to serve our shoppers with utmost performance for all occasions to come wanting ahead to serve you the very best quality devices and expert services+ Go through Additional
This way enables you to provide your information and talk to the Digital Dissolution Amount Test Apparatus vendor in regards to the most fitted and possible transportation method in your acquire. By partaking with the seller via this type, you'll be able to talk about and finalize the very best supply selections for you. Showcased Chart
Facts acquired from dissolution experiments generate choices and development all through formulation assisting to differentiate APIs (Energetic pharmaceutical ingredients), excipients, formulations, and manufacturing procedures on The idea in their ability to greatly enhance check here bioavailability.
Such data also assistance more and more advanced PK modelling to efficiently accelerate drugs to market read more place and assistance to safeguard quality through the entire life span of the drug, by means of generic advancement and without a doubt any transition to more than-the-counter provision.
 Jonathan Lipnicki Then & Now!
Jonathan Lipnicki Then & Now!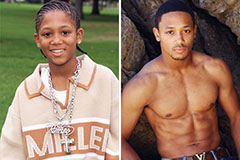 Romeo Miller Then & Now!
Romeo Miller Then & Now! Bug Hall Then & Now!
Bug Hall Then & Now!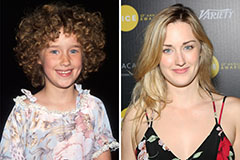 Ashley Johnson Then & Now!
Ashley Johnson Then & Now! Michael C. Maronna Then & Now!
Michael C. Maronna Then & Now!